Our intuitions are tuned to classical physics---the collection of physical laws and equations that govern the behavior of ordinary objects. For example, your car keys typically stay right where you put them an hour ago and don’t reappear in the refrigerator. It’s possible to plan an outfit the night before because your clothes don’t change color in the closet. And, notwithstanding the batter, a well-pitched baseball usually arrives at the catcher’s mitt and not in another stadium.
The
But in the quantum world---the world that emerges
In the quantum world, our intuitions about nature become less reliable. For one, quantum objects don’t have perfectly predictable motions---not even in principle. A
Quantum physics is slippery like this. A quantum object's position or speed can exist as a combination of possibilities until you measure it---that is, until you observe its location or how fast it's going. Once that happens, the combination vanishes, and position or speed can assume definite values. But as time goes on, those values tend to become uncertain again.
This innate uncertainty---and the accompanying probabilities---are core features of quantum physics. But there are other things that set the quantum world apart from the classical world.
One is illustrated by the difference between a ramp and a staircase. On a ramp, every spot along the way is fair game for taking a breather. But in the quantum world some properties can only have particular values, as though they were restricted to the steps of a staircase. You can stand on step 2, 3 or 4---and even with your feet on two different steps---
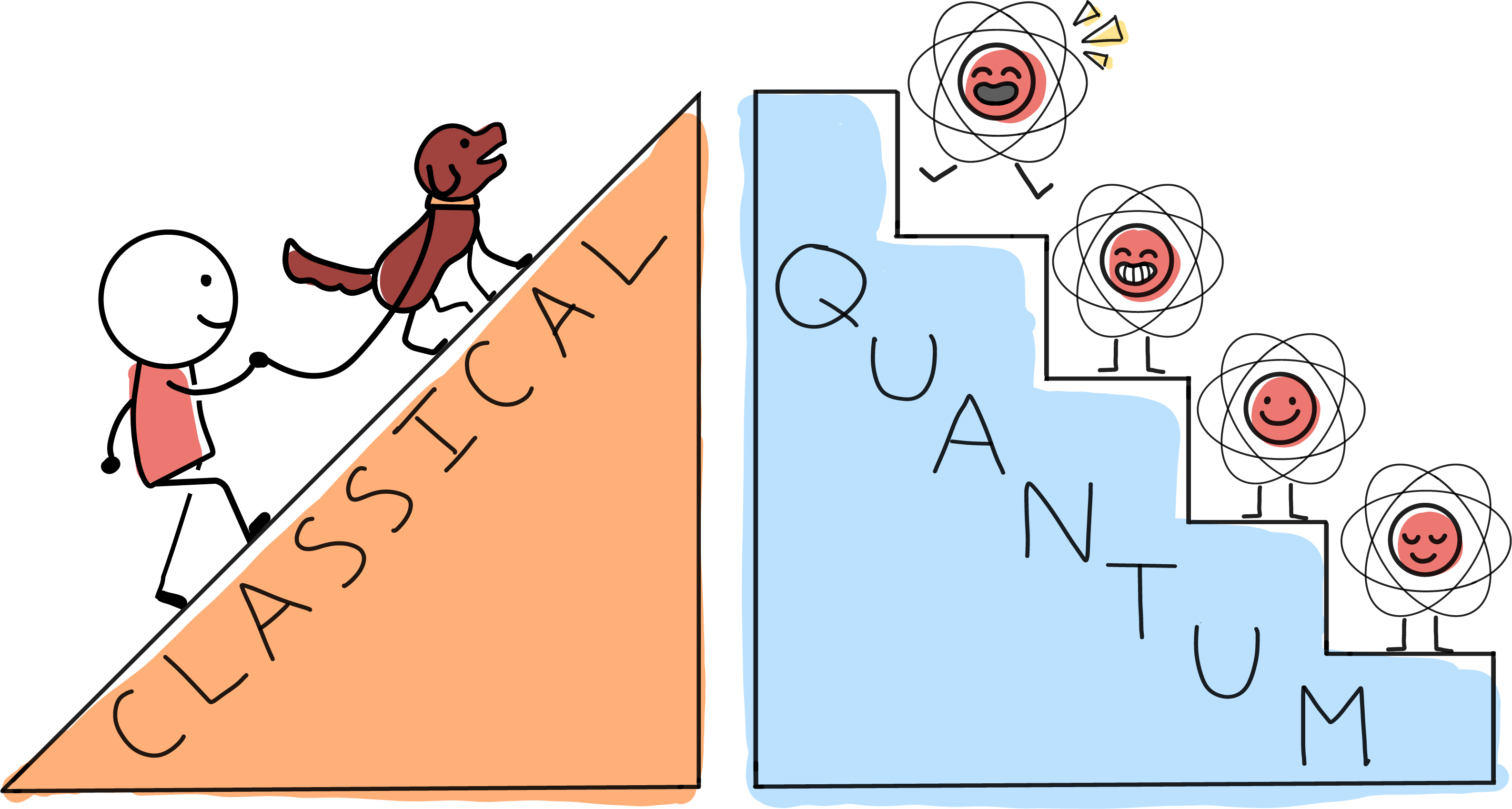
In the quantum world, energy becomes one of these quantized properties. For something large and classical, like a cat, there’s a ramp-like continuum of possible energies. But for tiny, quantum things, like the atoms making up the cat, there is a staircase of allowed values.
This staircase is what gives each element in the periodic table its own distinct structure, with a unique set of heights between each energy step---the distance between steps in a sodium atom is different than in a neon atom or an oxygen atom or any other element. Quantum physics predicts these patterns, which account for the different chemical and physical properties of all the elements.
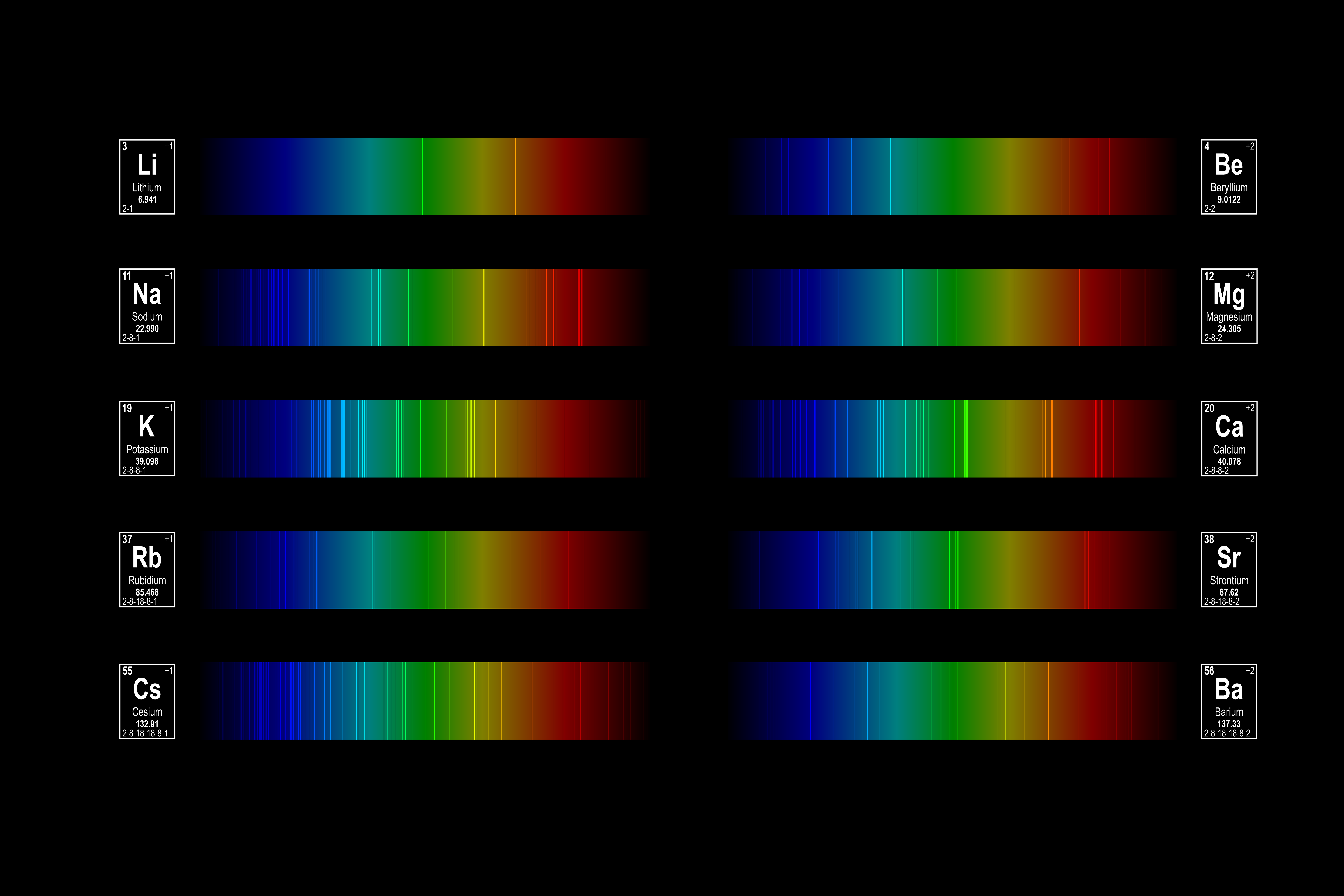
In fact, when an atom gains exactly the right amount of energy---no more, no less---one of its electrons can climb the staircase to a higher energy. Eventually that electron will fall back down, shedding the energy as a bundle of light---also known as a photon. The color of the photon depends on how far the electron falls, which means different elements will glow with different colors. Scientists can use these distinct colors like luminous fingerprints to identify elements from afar, allowing them to determine the composition of distant stars or galaxies.
Quantization is just one of the features of the quantum world that clashes with our intuition. There are many others, including wave-particle duality, entanglement, the uncertainty principle and spin---all of which have their own counterintuitive aspects. But although these features can be difficult to grasp, their predictive power is hard to deny: Together they form what is arguably the most well-tested description of nature that science has ever produced.







